
Acid-Base Chemistry
Acid-base chemistry can be found everywhere in our daily lives. It allows us to digest food, lift stains from laundry, and maintain healthy teeth through brushing. But how does it work?
In an acid-base reaction, two chemical species react by exchanging one or more hydrogen ions. These reactions are used to determine the concentration of a known analyte, neutralize a solution, or produce a specific product.
pH and pOH Scales
pH Scale
The pH scale is a standardized range of acidity measurements; in other words, it is a measure of how acidic a species is. A pH above 7 indicates alkalinity, and a pH below 7 indicates acidity, with the strength of acidity or alkalinity increasing as the value moves further from 7. A pH of 7 is considered neutral.
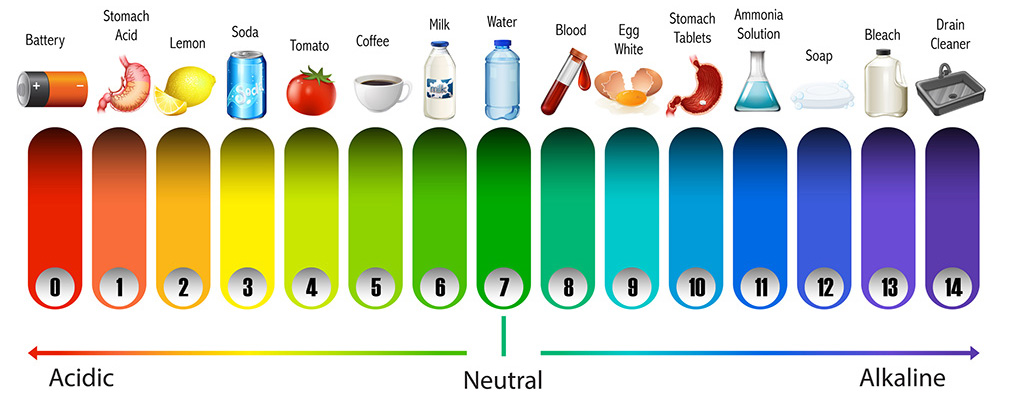
pH is defined as the negative logarithm of the concentration of hydrogen (H+) ions in a solution, as shown below:
$ \large \mathrm{ pH } = \mathrm{ -log }[H^+] = \mathrm{ log } \frac{1}{[H^+]} $
Because the pH scale is logarithmic, this means that one unit of pH represents a tenfold change in acidity or alkalinity. In other words, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4 (or contains ten times the concentration of hydrogen ions (H+) ions).
pOH Scale
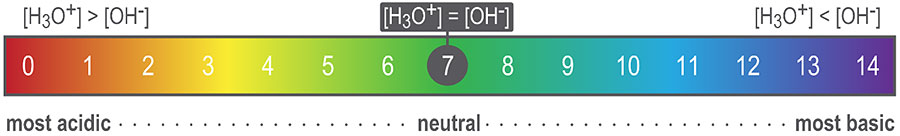
The pOH scale is used to measure how basic a species is. It reflects the concentration of hydroxide ions (OH-) in an aqueous solution.
pOH is defined as the negative logarithm of the concentration of hydroxide ions:
$ \large \mathrm{ pOH } = \mathrm{ -log }[OH^-] = \mathrm{ log } \frac{1}{[OH^-]} $
pOH and pH are related by the following equation:
$ \large \mathrm{ pH + pOH = 14 } $
Therefore, if we know the pH of a solution, we can calculate its pOH and vice versa. A solution with a pH of 7 is neutral, and its pOH is also equal to 7. For instance, at 298K (25°C), pure water is at equilibrium and contains equal concentrations (10-7M) of hydrogen ions (H+) and hydroxide ions (OH–).

pH and pOH are inversely proportional. When pH increases, pOH decreases by the same amount. A pH less than 7 indicates a relative excess of hydrogen ions, and the solution is considered acidic. The same is true when the pOH is greater than 7. Alternatively, a pH greater than 7 (or a pOH less than 7) indicates a relative excess of hydroxide ions, and the solution is considered basic. If both the pH and the pOH are equal to 7, the solution is neutral, meaning it contains equal concentrations of hydrogen and hydroxide ions.
Teaching Tools
Wireless pH Sensor
The Wireless pH Sensor is a must-have for any chemistry, biology, or environmental science course. Equally capable in the lab or field, the sensor eliminates the hassle of cables, reducing spills and improving safety. Plus, it rarely requires charging; the sensor's coin cell battery lasts for 2-3 years in most labs and costs about one dollar to replace. It can transmit data in real time, or store data for days when continuous monitoring is required. The Wireless pH Sensor enhances countless activities, including acid-base titrations, investigations into household chemicals, analyses of chemical reactions, water quality studies, and much more.
Buffers
A buffer is a solution that resists changes in pH when mixed with small amounts of a strong acid or strong base. Buffers, or buffer systems, consist of a weak acid and its salt (conjugated base + cation), or a weak base and its salt (conjugated acid + anion).
The two most common buffers consist of the following:
1) An acid buffer system composed of acetic acid (CH3COOH) and sodium acetate (CH3COO–Na+)
2) A basic buffer system made of ammonia (NH3) and ammonium chloride (NH4+Cl-)
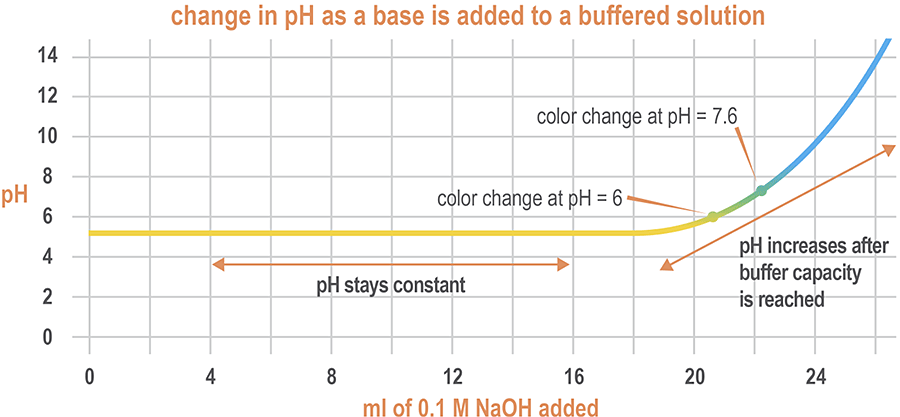
In the graph below, aqueous sodium hydroxide (NaOH) is added in small increments to a buffer solution. The buffer maintains a relatively constant pH until the 19-mL mark, at which point the pH of the solution begins to rise. Up until this point, the concentration of base is low enough to be buffered. After this point, the buffer’s capacity to resist changes in pH diminishes.
Buffers vary in their ability to resist changes in pH, a characteristic known as buffering capacity. The buffering capacity of a solution is a measurement of its ability to resist changes in pH.
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is used to estimate the pH or pOH of a buffer solution:
For a weak acid buffer solution:
$ \large \mathrm {pH} = pK_a + \mathrm {log} \frac {[A^-]}{[HA]} $
For a weak base buffer solution:
$ \large \mathrm {pOH} = pK_b + \mathrm {log} \frac {[B^+]}{BOH} $
Three Theories of Acid-Base Chemistry
Acid-base chemistry is defined by three primary theories: the Arrhenius theory, the Bronsted-Lowry theory, and the Lewis theory of acid-base reactions.
Arrhenius Acid and Base Theory
Developed in 1884, the Arrhenius Theory defines acids as hydrogen-containing compounds that dissociate to form an excess of hydrogen (H+) ions in water and bases as hydroxide-containing compounds that dissociate to form an excess of hydroxide (OH-) ions in water.
Arrhenius Acids
An Arrhenius acid is a hydrogen-containing compound that releases hydrogen ions when added to water, resulting in an increased concentration of (H+) ions in the solution. These hydrogen (H+) ions combine with water molecules to form the hydronium ion (H3O+), also known as the conjugate acid of water.
Common Arrhenius acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), hydrobromic acid (HBr), and hydrofluoric acid (HF).
Arrhenius Bases
An Arrhenius base is a hydroxide-containing compound that releases hydroxide ions when added to water, resulting in an increased concentration of OH- ions in the solution. Common Arrhenius bases include sodium hydroxide (NaOH), magnesium hydroxide (Mg(OH)2), and calcium hydroxide (Ca(OH)2).
Limitations of the Arrhenius Theory for Acid and Bases
The Arrhenius theory was the first to be developed, and it’s considered the simplest of the three theories for acid-base chemistry. While some basic compounds do release hydroxide ions in water, others do not. According to the Arrhenius theory, acids dissociate to increase the concentration of hydrogen (H+) ions in a solution, while bases dissociate to increase the concentration of hydroxide (OH-) ions in a solution. So how do we explain the behavior of compounds without hydroxide ions, like F- and NO2-, which act as bases when added to water? We turn to theory number two, Bronsted-Lowry.
Bronsted-Lowry Acid and Base Theory
The Bronsted-Lowry theory explains acid-base reactions by following the transfer of protons between chemical species. It describes acids and bases in terms of their ability to accept or donate protons.
Bronsted-Lowry Acids
A Bronsted-Lowry acid is a proton donor, or a chemical species that can donate a proton (H+) to another chemical species. All Bronsted-Lowry acids can also be classified as Arrhenius acids.
Common Bronsted-Lowry acids include nitric acid (HNO3), sulphuric acid (H2SO4), hydrochloric acid (HCl), and hydrofluoric acid (HF).
Bronsted-Lowry Bases
A Bronsted-Lowry base is a proton acceptor, or a chemical species that can accept a proton (H+) from another chemical species. Compounds classified as Bronsted-Lowry bases cannot be assumed to be Arrhenius bases.
For example, NaOH (sodium hydroxide), NH3 (ammonia), and F- (fluorine) are all Bronsted-Lowry bases, but only NaOH is both an Arrhenius base and a Bronsted-Lowry base. NH3 and F- are not Arrhenius bases because they do not dissociate into hydroxide (OH-) ions in aqueous solutions.
Arrhenius Bases vs. Bronsted-Lowry Bases
The Bronsted-Lowry theory helps fill some of the gaps in the Arrhenius theory, which falls short of explaining the basic behavior of compounds without hydroxide (OH-).
For example, when the basic salt, sodium fluoride (Na+F-), is added to water, the concentration of hydroxide ions present in the solution increases. Under the Arrhenius theory, this does not make sense, as Na+F- does not contain hydroxide. But under the Bronsted-Lowry theory, it becomes clear that the reaction is driven by proton exchanges. The basic salt, sodium fluoride, acts as a weak base and accepts protons from the surrounding water molecules. As a result, an excess of hydroxide ions form.
Conjugate Acid-Base Pairing
In Bronsted-Lowry reactions, the protons are transferred from the acid to the base. Because of this, Bronsted-Lowry acids and bases occur in pairs, a phenomenon known as conjugate acid-base pairing.
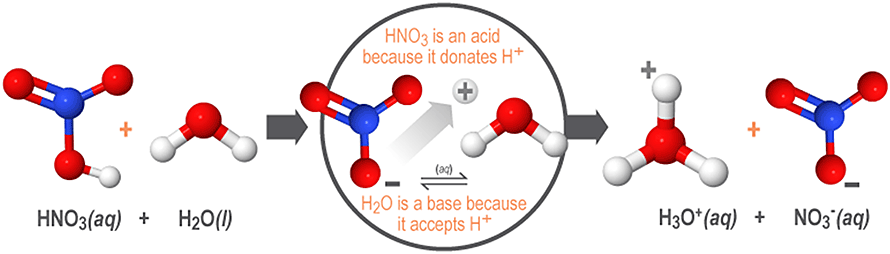
For example, when dissolved in water, nitric acid (HNO3) donates protons (H+) to the surrounding water molecules. This produces its conjugate base, the nitrate ion (NO3-). As shown below, water acts as a base by accepting these protons and forming its conjugate acid, the hydronium ion (H3O+).
Lewis Theory of Acids and Bases
The Lewis theory of acid-base reactions classifies acids and bases by their ability to accept or donate pairs of electrons.
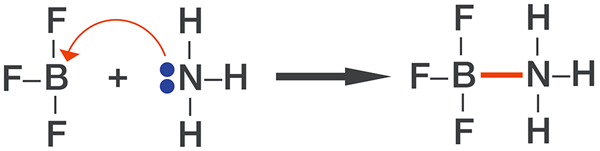
When a Lewis acid-base reaction occurs, the Lewis acid accepts a pair of non-binding electrons from the Lewis base. The Lewis acid is an electron-pair acceptor, and the Lewis base is an electron-pair donor.
When a Lewis acid and a Lewis base react, a coordinate covalent bond forms between them, and a special compound, known as a Lewis acid-base adduct, is formed.
Tips for Classifying Acids and Bases According to Theory
A good rule of thumb to remember about acids and bases is that every Arrhenius acid or base can be classified as a Bronsted-Lowry acid or base, and every Bronsted-Lowry acid or base can be classified as a Lewis acid or base. However, these rules do not apply in the opposite order.

Amphoteric Species
An amphoteric species is a molecule that can act as both an acid and a base. The most common amphoteric solutions are water, aqueous ammonia, and aqueous di- or triprotic acids and bases. In an acidic environment an amphoteric species acts as a base, and in a basic environment it acts as an acid. An often-used example is water because it can act as either an acid or a base depending on the solvent it is reacting with.
Water behaves as a base when reacting with an acid.
$ \large \mathrm { HA + H_2O \rightleftharpoons H_3O^+ + A^- } $
Water behaves as an acid when reacting with a base.
$ \large \mathrm { H_2O + B^- \rightleftharpoons HB + OH^- } $
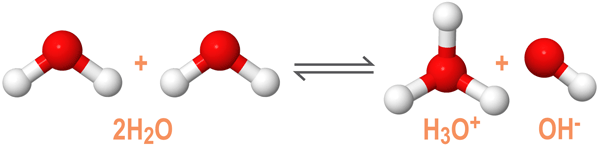
Autoionization of Water and the Autoionization Constant
As an amphoteric compound, water reacts with itself through a process called autoionization or self-ionization. The autoionization reaction of water is shown below:
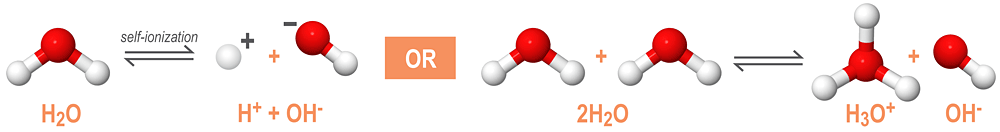
In a solution of pure water, two water molecules will react to produce equal concentrations of hydronium (H3O+) and hydroxide (OH-) ions. At room temperature (25ºC), the molarities of the hydronium and hydroxide ions are both equal to 1.0 x 10-7M. The autoionization constant of water, Kw, is used to describe the equilibrium conditions required for the self-ionization of water.
At room temperature, the product of the molarity of the hydronium and hydroxide ions is equal to 1.0 x 10-14M. Thus, Kw can be determined using the expression:
$ \large \mathrm { K_w } = [H_3O^+] [OH^-] = 1.0 \times 10^{-14} $

